
Fuel cells, in general, are units that convert chemical energy found within various fuel sources along with an oxidizing agentinto electricity through a pair of redox reactions. Some are capable of taking electricity, water, and gas and converting these inputs into hydrogen. This paper covers the fundamentals of the various fuel cells in use today. It also mentions some of the places they may be found based on their operating parameters and capabilities.
Types of fuel cells currently on the market:
Polymer Electrolyte Membrane Fuel Cells (PEMFC) AKA Proton Exchange Membrane Fuell Cells
– Consists of (2) catalyst electrodes separated by polymer electrolyte
– These involve a platinum nanoparticle cathode and anode catalysts which are deposited onto a carbon support material
– Operate at lower temperatures which results in less water on system components, increasing durability
– Fast start-up time
– Short response time
– Low gas cross permeation
– Higher purity of hydrogen produced
– Higher thermodynamic voltage
– Can withstand higher operating pressure across the internal membrane
– Low ohm loss
– Operate over a wide range of power inputs
– Lower operational costs
– Electrical efficiency is 40%–50%,
– Power capacity at output is up to 250 kW.
Disadvantages
– High cost
– Limited choices of stable earth-abundant electrocatalysts
– Easily damaged by inappropriate operation
– Sensitive to imperfections, dust, and impurities
– Low-temperature water for heating
– Critical on gas quality
Where might these be seen:
– Great candidate for powering automobiles
o passenger vehicles, forklift and material handling, trains
– portable power supply applications
o laptops, cell phones
– local power generation to support homes and businesses
– Replacement of portable diesel generators
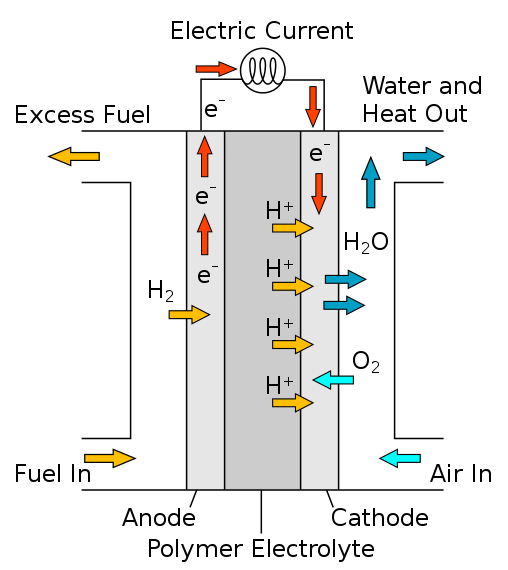
Diagram of a Proton exchange membrane fuel cell.
Albris, Public domain, via Wikimedia Commons
Direct Methanol Fuel Cells (DMFC)
Disadvantages
Where might these be seen:
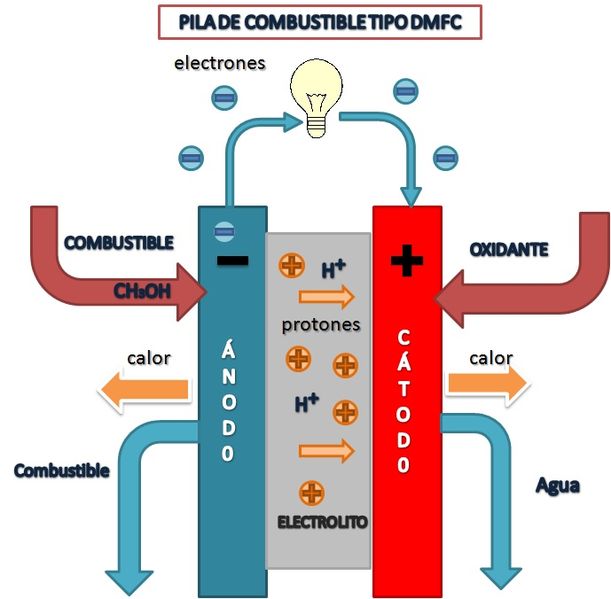
DMFC type fuel cell diagram
Amalia1983, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
Alkaline Fuel Cells (AFC)
Disadvantages
Where might these be seen:
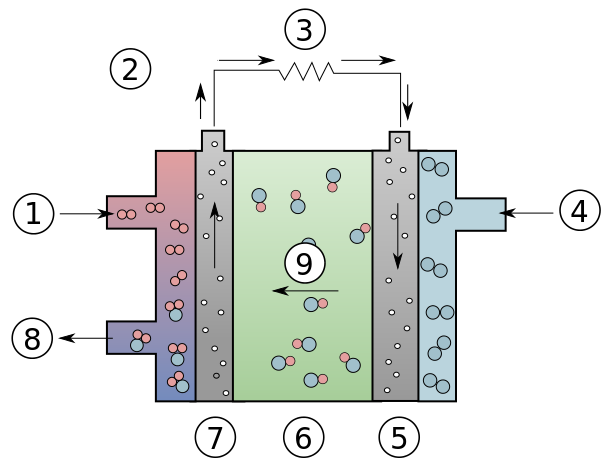
Alkaline Fuell Cell.
1: Hydrogen 2:Electron flow 3:Charge 4:Oxygen 5:Cathode 6:Electrolyte 7:Anode 8:Water 9:Hydroxyl Ions
Created by Darryl Ring, Vectorization: Chabacano, CC BY-SA 3.0 http://creativecommons.org/licenses/by-sa/3.0/>, via Wikimedia Commons
Phosphoric Acid Fuel Cells (PAFC)
Disadvantages
Where might these be seen:
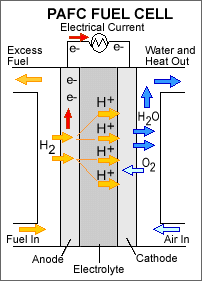
Phosphoric acid fuel cell
U.S. Department of Energy, USA.gov, Public domain, via Wikimedia Commons
Molton Carbonate Fuel Cells (MCFC)
Disadvantages
Where might these be seen:
Output in the 100 kW to 400 kW range
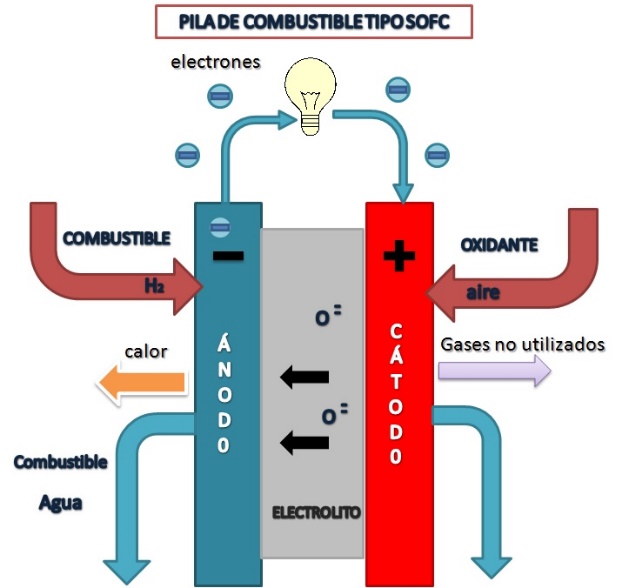
MCFC type fuel cell diagram
Amalia1983, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
Solid Oxide Fuel Cells (SOFC)
Disadvantages
Where might these be seen:
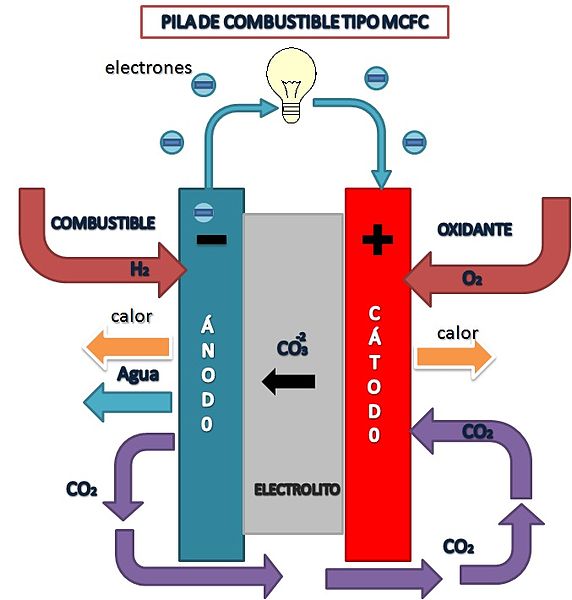
SOFC type fuel cell diagram
Amalia1983, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
If you are considering implementing fuel cells to meet your energy needs, Ryanco Construction can help you choose, design,and install the right system. We work with suppliers of various fuel cells to fit your project needs and constraints.


Want to work together? Email us at ryan@ryancoconstruction.com
